Difference between revisions of "T07 Pensky-Martens Closed Cup Tester"
From Chemical Engineering @ UP wiki
| Line 1: | Line 1: | ||
| − | [[File: | + | [[File:tribology1.jpg|right]] |
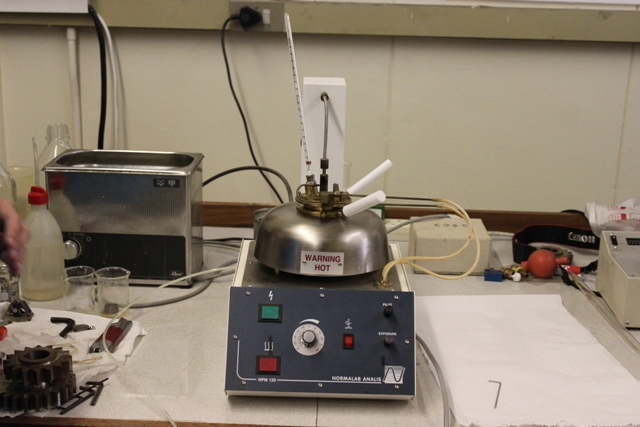
The flashpoint of a substance is the lowest temperature at which it will ignite and momentarily burn when exposed to an ignition source such as a naked flame. | The flashpoint of a substance is the lowest temperature at which it will ignite and momentarily burn when exposed to an ignition source such as a naked flame. | ||
Being aware of the flashpoint of a volatile substance is crucial in order to improve general safety procedures in the laboratory and to prevent potential fires and explosions. | Being aware of the flashpoint of a volatile substance is crucial in order to improve general safety procedures in the laboratory and to prevent potential fires and explosions. | ||
Revision as of 08:15, 7 December 2011
The flashpoint of a substance is the lowest temperature at which it will ignite and momentarily burn when exposed to an ignition source such as a naked flame. Being aware of the flashpoint of a volatile substance is crucial in order to improve general safety procedures in the laboratory and to prevent potential fires and explosions. Knowledge of the flashpoint of a diesel will also enable one to ascertain whether or not it will combust in a particular diesel engine.
Documentation
Test Procedure
The Pensky-Martens Closed Cup Test Procedure was compiled by S.C. Rencken during December 2011